IBBR Request for Proposals (RFP): GMP Biomanufacturing Facility Available – Rockville, Maryland, USA
Published 8/19/19 on the PR Newswire
ROCKVILLE, Md., Aug. 19, 2019 /PRNewswire/ — The Institute for Bioscience and Biotechnology Research (IBBR) is interested in proposals to lease a biomanufacturing facility configured to produce biologicals under cGMP conditions in compliance with FDA requirements for phase I/II clinical trials. The facility is also equipped to perform process development research, pre-clinical manufacturing for material necessary to conduct IND-enabling toxicology studies, Point of Concept (POC) studies, and process demonstration in advance of Good Manufacturing Practices (GMP) manufacturing. IBBR is a joint research enterprise between the University of Maryland and the National Institute of Standards and Technology.
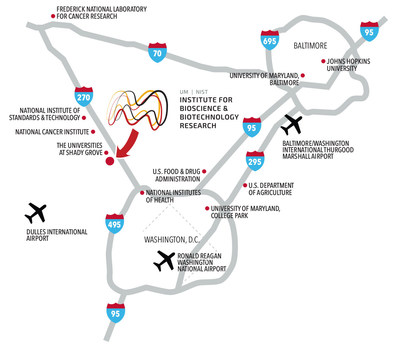
Location: IBBR is strategically located just northwest of Washington, DC, in the heart of Maryland’s biotechnology corridor, within easy reach of major academic, industry, and federal research laboratories, including University of Maryland campuses, NIST, NIH/NCI, FDA, and USDA. The GMP facility is located on IBBR’s campus at 9600 Gudelsky Drive, Rockville, MD 20850.
Deadline: IBBR is requesting proposals for review on a case-by-case basis until a preferable and credible response is identified before September 30th, 2019.
Proposals will be evaluated based upon the following criteria:
- Qualifications of applicant and personnel to be engaged in manufacturing activities.
- Rental rate proposed to IBBR.
- Type of collaboration proposed (simple lease, revenue sharing, other).
- Use of facility, if necessary, for manufacturing clinical product candidates that might be developed by IBBR and the University of Maryland.
- Potential involvement with research and training and education programs currently conducted at the institute. Please visit www.ibbr.umd.edu for more information on research areas of focus.
In addition, IBBR will consider proposals favorably based on projected economic development impact, community benefit, research collaboration with IBBR and the University, quality and nature of any proposed capital improvements to the GMP facility as well as other factors whereby the successful applicant’s use of the GMP facility supports the University of Maryland’s educational and economic development mission. IBBR reserves the right to not select any proposal.
Facilities and Services Available:
- Approximately 9,100 square feet of GMP suite, laboratory space, support areas, and offices.
- Designed and configured for bacterial or yeast fermentation and mammalian cell culture to manufacture products such as therapeutic proteins, CAR-T cell therapies, monoclonal antibodies, vaccines, cytokines, DNA plasmid vaccines, or nanotechnology based products.
- Flexible configuration that can be modified as per specific needs of the applicant.
- Infrastructure support such as utilities, maintenance contracts, and facilities management.
Guidelines
Proposals should include:
- Electronic PDF submission of proposal.
- Lease amount inclusive of utilities per square foot per annum.
- Financials regarding other types of collaborations (if applicable) including revenue forecasts
- Proposed use of the facility.
- Economic Development Impact (i.e. relocating or creating jobs in the area).
- Preference for the manufacture of product candidates developed by IBBR and University of Maryland.
- Other factors related to IBBR’s scientific mission and vision.
Other Considerations:
- All costs, proposed investments, contributions, and financial expectations related to the proposal should be outlined.
- Upon identification of a satisfactory proposal, the parties will develop a lease reflecting the terms of the proposal.
- Total lease term not to exceed 10 years including renewals.
- All information related to this RFP and submitted by applicants will be confidential.
For additional information and to schedule a tour of the GMP facility, please contact:
Viqar Aslam
Director Business Development and Strategy
9600 Gudelsky Drive
Rockville, MD 20850
Phone: 240-314-6273
Email: vaslam@umd.edu